Friday, March 31, 2006
Streamlining of links
So its not so cluttered on my links section. The link to the new index page is placed under.. the links section of cse.. where else?
However.. you still have to surf in to my blog for any NEW notes. I will use that index page only for old notes (maybe a term by term update)
hope everyone has a nice weekend!
Thursday, March 30, 2006
Run
well at least she gets a shiny new bum FOC. but in the meantime, i think i need to crawl for another few days. UGH.
oh it seems that J8 is crawling with 3N pple.. bumped into a few of them XD
and looks like every1's quite busy. I stayed late in school to help run a perfumery worskshop.. its pretty fun! we got to extract essential fragrance from some fruits.... maybe someday we can try that for flowers and other stuff blend our own perfumes. but really, extraction from nature gives such a low yield.. its far better to obtain the fragrances directly through organic synthesis from cheap raw materials. after workshop had to do some admin and datamanagement.. and before i knew it its 8pm. I think i need an extra 2 hrs a day.
chilling out to snow patrol now.. its so emo haha!
here's my fav:
"Run" - Snow patrol
I'll sing it one last time for you
Then we really have to go
You've been the only thing that's right
In all I've done
And I can barely look at you
But every single time I do
I know we'll make it anywhere
Anyway from here
Light up, light up
As if you have a choice
Even if you cannot hear my voice
I'll be right beside you dear
Louder louder
And we'll run for our lives
I can hardly speak I understand
Why you can't raise your voice to say
To think I might not see those eyes
Makes it so hard not to cry
And as we say our long goodbyes I nearly do
Light up..
louder...
Slower slower
We don't have time for that
I just want to find an easier way
To get out of our little heads
Have heart my dear
We're bound to be afraid
Even if it's just for a few days
Making up for all this mess
Monday, March 27, 2006
healthy lifestyle!
eh... actually it has to do with pheobe.. she just got her rear end hurt...
.. oh err yea pheobe's my CHIO red car! it all started with this cab driver..... couldnt wait to kiss pheobe's a**
ugh.. too tired to blog now... i only slept for like 2 hrs (4 am to 6 am today) after working on my entire sunday.
cannot take it oredi..
oh yes.. pls go get the cehm bonding discussion k! btw, the table on page 8 is like a sumamry to chem bonding in itself, so use it.
ciao.
Sunday, March 26, 2006
Chem bonding Discussion
Its like a summary of key points and perspectives, except that it is expanded to include Key explanations.
-->Click here<-- to get it
A real summarized summary will come soon
Chem lecture test 1.
But don't be discouraged, most likely if one of the items below are improved, you'd soon find chem a breeze.
1. approach, perspective and method of learning and problem solving
- some think that chem is mysterious, cheem or cannot be visualized
- some try to memorize without understanding. this leads to pt 2
2. confusing between too many facts and points
- break down complex problems/observations link each observation to the main causative factors.
- examining factors 1 by 1 to solve problem/exaplain observation
- have a systematic methodology (like step by step mtd) to solve problems
- summarizing all the above 3 points into gd summaries for each topic
3. Experience and mastery. comes with practice and application of pt 2 above into regular problem solving situation.
in short, some need a paradigm shift of how they treat the subject(esp those that find chem senseless or mysterious), some need problem solving skills(those that read a qn and dunno whats expected of them), and most need experience and practice.(those that out of time, miss key points, careless)
Thursday, March 23, 2006
agony anonymous!
Wow so many anonymous taggers on my board... pls dun be anon next time.
alright i get the point of these students. I'm sorry, i didnt mean to say that ALL students who did badly have bad attitude or didnt try or don;'t care. I know there are those who studied and those who tried.
Right let me be more specific.
1. In each class there are always some students who are truly lazy/illdisciplined/ dun care. How'd I know?
I observe. These fellas always :
a) hand in late work,
b) when i go around tutorial during discussion they have not prepared, but instead have a blank piece of paper.c)
c) when i discuss points in class, they'd stared blankly.
not bothering to:
-process respond, clarify doubts, paritcipate
-take dopwn important key points and notes
-usually when the bell goes but i'm not ready to dismiss yet, these pple will pack their bags while i'm still discussing something! amazing!
2. Those who do not respond in class.
while this is not a "bad" attitude, its an undesirable one. they just get lost.. and keep quiet about it. How to learn?
3. Those who study AT THE LAST MINUTE and not consistently trying/understanding/following
Last minute work doesnt cut it. busy during the hols?? come on so was I and every1 else. manage your time. if you work consistently during term time, your wouldnt have to do last minute work, or expend too much of your holidays. its only 3 topics. all u need is about 3 hrs for 3 days and you're pretty much done. DO NOT tell me you cannot spare 9 hrs of your 1 week holiday, plus the fact that these things shd be clear during the term itself.
4. certain people refuse remedial when i asked them if they wanted it. too bad.
5. certain people never visit this blog and they don't bother to get the notes. too bad also.
6. corrections, see me's....
some people refuse to learn from their mistakes. they don't mark their work properly when we go through or comparing with suggested solutions, they don't do corrections. Some people don't bother to see me when i write see me in their work.
7. people who got 5 marks or less.
how's that even possible unless they didnt study?!?! are they that inept?? I think its an insult to say that 5 marks is their standard.
So there, if you do not belong to on of the 7 categories above. I don't mean to scold you. When i admonish students, especially for common issues, I have to admonish the class together. but i know who are the real black sheep. So if you've been really trying, you have my respect, and know that my admonishings are not for you.
If you've been reaaally trying and dint do well, perhaps you need to reflect on why is that the case, and maybe to see me to discuss some solutions.
If you belong to any1 of the 7 categories above, please accept the criticism and improve.
so there i still love all of you k! (perhaps thats why its painful to see the horrible results and the above probs 1-7, if I dun care, i'd be unaffected)
oh btw about the notes. At the very least I will not give out the chembonding 1 assigment worked solutions. why?
because on wednesday, in a certain class, there were these 2 students who refused to mark their work as i went through the solutions in class. Even when i told them directly, twice, to do so. And all they had was their bag(packed up and eager to leave) in the desk.. no work, nothing, not taking down anything. this was on wednesday 22/3/06 11-12 noon.
I will not let these bochap students benefit without putting in effort. Therefore no worked solutions will be released. too bad.
Wednesday, March 22, 2006
Chem notes
highlight main points in your notes.
For summaries, 1st read the notes, understand the concepts and jot those concepts down beside the sumarized key points
Anyway, once I complete chem bonding 1 and 2 summary, I will not make or post further notes, since so many pple have a bochap attitude.
Tuesday, March 21, 2006
Every girl should have a....
And its a community service message! remember: EARLY DETECTION SAVES LIVES!
here are the details:
About The Project
We are supporting the Breast Cancer Foundation - specifically to help them to increase awareness of and knowledge on breast cancer and related issues amongst young women aged 20-39 yrs. Our focus is to encourage young women to make Breast Self Examination (BSE) a part of their lives from a young age, as this is the best way to protect one's self.
To aid us in this endeavour, we will be selling custom-made planners that include suggestions and information about how women can protect themselves.
Dates: 20th - 22nd March (Mon-Wed)
Venue: SMU, The Concourse (outside Kopitiam)
BCF Notebooks with Planner
Our team has specially designed custom-made BCF planners which includes a Monthly Planner, reminding women to examine themselves on particular dates of the month. The planners will contain facts and messages from the BCF including, how to do BSE, myths and truths about breast cancer and the various know causes of breast cancer, to name a few. The highlight of the planner is the calendar as it will contain periodic reminders to the owner to do BSE on particular days. The planner will also include a few inspirational stories from breast cancer survivors about their struggle with and victory over the disease.
These notebooks are sold at a minimum donation of $5. All proceeds will go to the BCF.
Guys, you can buy the pink book for your loved ones too! Like your mum, aunties, sisters, girlfriends etc etc!
Monday, March 20, 2006
super secret studying technique
cos there's a *super secret studying technique* teehee.. teach you k
Ok its based on a chem and bio idea. We know that stuff will diffuse from a region of high concentration to low concentration right?
Assumptions: the notes contain more knowledge than our brains k.
right so when you sleep you put the notes under ur head. or maybe on top of it (but it may slip off..) during the 4-8 hrs that you are asleep the knowledge will diffuse into your brain! In fact, I actually dreamed of studying some stuff before... and the qns came out for exams before! (ok i was really desperate)
Heh this technique will definitely save you time so that you can surf really cool blogs such as this one :)
super secret studying technique
cos there's a *super secret studying technique* teehee.. teach you k
Ok its based on a chem and bio idea. We know that stuff will diffuse from a region of high concentration to low concentration right?
Assumptions: the notes contain more knowledge than our brains k.
right so when you sleep you put the notes under ur head. or maybe on top of it (but it may slip off..) during the 4-8 hrs that you are asleep the knowledge will diffuse into your brain! In fact, I actually dreamed of studying some stuff before... and the qns came out for exams before! (ok i was really desperate)
Heh this technique will definitely save you time so that you can surf really cool blogs such as this one :)
Chem Bonding 1 Summary
Its meant to be complete and seamless with chembonding 2. so when that's about done, i'll do the summary for chembonding 2 too. So please file up the whole thing together then.
so ----> CLICK HERE <------
Saturday, March 18, 2006
I wanna visit Christmas Island!!
we can try steamed, chilli, black pepper, butter fried, temakied, teriyakied, BBQ.. omg there are enuff to go around for every recipe in the book.
:) :) :)
Mid life angst
i mean 1/8 of the jc life of this batch is over! and things are only gonna get more intense. and then all of a sudden they will graduate and disappear... ~~sigh~~. kk before this little bout of angst morphs into a really morose dispirited wail of resignation.. i will.. get a haircut!!! Hair Inn at centre point very nice!! :) :) heh cut and wash for only 20$ ++ nice service, good cut, and a really comfy wash.. d*** therapeutic i tell you!
that plus i actually do need a haircut for that mop thats growing on my head.
ohoh and plus some Robinson's vouchers i have to spend :D
oh haha those jokes in my previous postings (if you didnt get them, they are jokes ok.. so LAFF!) they are not originally from me of course. some of you might have seen it before. I just happened to surf onto them again and decided to do a little cut n pastin.
heh later on i will talk about really funny bio and chem stuff i just read. Like Trombicula fujigmo and cummingtonite.
but yes back to angsty stuff....

pic is (c) Kurt Halsey
check out Kurt Halsey
really nice angsty stuff.
Friday, March 17, 2006
Beware of Dihydrogen Monoxide: A dangerous chemical!
Dihydrogen monoxide is colorless, odorless, tasteless, and kills uncounted thousands of people every year. Most of these deaths are caused by accidental inhalation of DHMO, but the dangers of dihydrogen monoxide do not end there.
Prolonged exposure to its solid form causes severe tissue damage. Symptoms of DHMO ingestion can include excessive sweating and urination, and possibly a bloated feeling, nausea, vomiting and body electrolyte imbalance. For those who have become dependent, DHMO withdrawal means certain death.
Dihydrogen monoxide:
· is also known as hydroxl acid, and is the major component of acid rain. · contributes to the "greenhouse effect." · may cause severe burns. · contributes to the erosion of our natural landscape. · accelerates corrosion and rusting of many metals. · may cause electrical failures and decreased effectiveness of automobile brakes. · has been found in excised tumors of terminal cancer patients.
Contamination is reaching epidemic proportions!
Quantities of dihydrogen monoxide have been found in almost every stream, lake, and reservoir in America today. But the pollution is global, and the contaminant has even been found in Antarctic ice. DHMO has caused millions of dollars of property damage in the midwest, and recently California.
Despite the danger, dihydrogen monoxide is often used:
· as an industrial solvent and coolant. · in nuclear power plants. · in the production of styrofoam. · as a fire retardant. · in many forms of cruel animal research. · in the distribution of pesticides. · as an additive in certain "junk-foods" and other food products.
Even after washing, produce remains contaminated by this chemical.
Companies dump waste DHMO into rivers and the ocean, and nothing can be done to stop them because this practice is still legal. The impact on wildlife is extreme, and we cannot afford to ignore it any longer!
The American government has refused to ban the production, distribution, or use of this damaging chemical due to its "importance to the economic health of this nation." In fact, the navy and other military organizations are conducting experiments with DHMO, and designing multi-billion dollar devices to control and utilize it during warfare situations. Hundreds of military research facilities receive tons of it through a highly sophisticated underground distribution network. Many store large quantities for later use
Thursday, March 16, 2006
German rocks!
The European Commission has just announced an agreement whereby English will be the official language of the EU rather than German which was the other possibility.As part of the negotiations, Her Majesty's Government conceded that English spelling had some room for improvement and has accepted a five year phase-in plan that would be known as "Euro-English".In the first year, "s" will replace the soft "c". Sertainly, this will make the sivil servants jump with joy. The hard "c" will be dropped in favour of the "k". This should klear up konfusion and keyboards kan have 1 less letter.There will be growing publik enthusiasm in the sekond year, when the troublesome "ph" will be replaced with "f". This will make words like "fotograf" 20% shorter.In the 3rd year, publik akseptanse of the new spelling kan be ekspekted to reach the stage where more komplikated changes are possible. Governments will enkorage the removal of double letters, which have always ben a deterent to akurate speling. Also, al wil agre that the horible mes of the silent "e"s in the language is disgraseful, and they should go away.By the fourth year, peopl wil be reseptiv to steps such as replasing "th" with "z" and "w" with "v". During ze fifz year, ze unesesary "o" kan be dropd from vords kontaining "ou" and similar changes vud of kors be aplid to ozer kombinations of leters.After zis fifz yer, ve vil hav a reli sensibl riten styl. Zer vil be no mor trubl or difikultis and evrivun vil find it ezi to understand ech ozer. Ze drem vil finali kum tru! And zen world!
Tuesday, March 14, 2006
March holidays remedial ROUND 2A
Dats right.. i'm asking for it.. remedial round 2 for 3R! The usual suspects pls turn up. and it wil be thursday 8 am B33.
*Sigh* I'm so sad I didn't really get to join the archery camp properly cos i got all these remedials and sci pjts and whatchamacalit commitments.
Mr Wong Is masochistic.
Hehe but did i tell you I'm sadistic too? wait till we set exam papers... TEEHEE
Friday, March 10, 2006
Chembonding Assignment for ALL students
March holiday remedials
Topics covered will be Atomic structure as well as Chembonding 1.
So please read all your notes fromthe college and then read the notes I've posted online for you to download, and bring them and bring your tutorials along.
Also please download remedial assignment for atomic structure and chembonding1 and attempt them before coming for the remedial.
Holiday remedial schedule:
3R: Tuesday 2-4pm B33
Kelly
Alamelu,
Angie Goh
Dharshni
Crystal
Shuyu
Natalie Chang
Natalie Low
7B: Wednesday 8-10 am b33
Shirong
Tania
Sherine
Neeti
Chengwei
Siqi
Ruolin
3N: To confirm a day when term starts. for 2hr slot
Abigail
Terese
Juee
Noraisha
Emelyne
Remedials and Consultations
Tues 4-6
Wed 9-12, 2-4
Thurs 10-2, 4-6
Fri 11-2
Sat 8-10
Subject to availability and prior appointments need to be made. Please feel free to make appointments with me to clear up your doubts. Be warned that you need to prepare your work adequately before you see me (this applies to lessons, remedials, and consultations)
Thursday, March 09, 2006
Roll of Honour - AS Quiz
As promised, I'm going to reveal the names of those who did well (scored 80% and above) and their scores in the AS quiz, and also some class statistics(A grade is 25marks and above) so that we can honour their efforts and achievement.
07S03H
1. Teo Si Rui 35/35
2. Lawrence Wu 35/35
3. Kenny Lim 33/35
4. Tabitha Quake 33/35
5. Nazirul 31/35
6. Michelle Chua 30/35
7. Amanda Lai 29/35
8. Samuel Wong 29/35
9. Benjamin Low 28/35
10. Christopher Tang 28/35
11. Vivek 28/35
S03H class average score 26.3
S03H class %A : 70%
07S03N
1. Adam 31/35
2. Kristel Low 30/35
3. Audrey Ng 30/35
4. Subash 29/35
5. Hyqel 28/35
S03N class avg score 19.7
S03N class %A : 24%
07S03R
1. Lee Xin Hui 33/35
2. Teo Yi Lyn 33/35
3. New Jin Rou 32/35
4. Lee Hwee Juin 31/35
5. Joshua Lian 31/35
6. Tan Shimin 29/35
7. Matthew 29/35
8. Toh Jia Yi 29/35
9. Bibianna Yeo 29/35
S03R class avg score 24.8
S03R class %A: 63%
07S07B
1. Chen Yanheng 30/35
S07B class avg score 18.3
S07B class % A 13%
Wednesday, March 08, 2006
Important Final Discussion on Atomic Structure
I've bursted my brains compiling all the atomic structure clarification questions not answered during turorials. That was quite abit of work so do take the time to look through them,; you may find interesting nuggets there.
Finally i want to discuss something important which I didnt touch on in tutorials very much. That is the electronic configurations of Cr and Cu.
While your notes have all the details, I want to highlight the key points to you
A) Cr is [Ar] 3d5 4s1 and not [Ar] 3d4 4s2
Why? because:
1. 4s and 3d orbitals have comparatively similar energy. So electron residing in either orbital will have not much energy difference
2. So the 4s electron is transferred to 3d orbital to minimize repulsion; 4s2 electronic configuration has paired electron whereas for 4s1 3d5 electronic configuration has no paired electrons. This is a sort of application of Hund's rule
B)Cu is [Ar] 3d10 4s1 and not [Ar] 3d9 4s2
Why? because
1. 3d10 electronic configuration has all d orbitals fully filled. resultant d subshell has a symmetrical distribution of charge which confers extra stability.
Finally. I have posted up some notes, the links are on the "links" section ---->
Atomic Structure Clarification Questions Part Deux
A: Yes! In fact the f block elements start at lanthanum in period 6. That means 4f is filled after 6s.
Q2. What are the properties of plasma?
A: What a big question. You can read all about plasmas here.
Q3 What does IE graph of ionization energy after the 3r IE look like?
A: Go and get the data booklet from SEAB. This is part of the exam syllabus and provides the 4th IE of many elements. You can use that to plot a graph and see for yourself. The 4th IEs and all follow the same basic rules. Refer to Qn25 in my earlier post.
Basically IE increase across the period due to Zeff. Trhe irregularities due to s vs p subshell or paired vs unpaired electrons may still be there.
You should also note that the elements having inner shell electrons removed will have high IE due to large Zeff(which is due to the smaller shielding in inner shell electrons)
Q4. If quantum nos are not important, do we need to know all these?
A: Its cause they are the basis for our knowledge of atomic structure
Q5. Why is 4s filled before 3d? If it is a 4th shell how can it be closer to the nucleus?
A: 4s is filled before 3d because it is lower in energy.[ 4s shell is still LARGER than 3d orbitals. But its shape makes 4s orbitals more PENETRATING and allows the electrons to move nearer the nucleus ON THE AVERAGE. This is what makes the 4s orbital lower in energy. The maximum boundary and distance of a 4s electrn is further from the nucleus than 3d electrons]
Q6. Are there any exceptions to the 3 rules for determining electronic configuration?
A: Yes note the electronic configs for Cr and Cu.
Q7. What does the f-orbital look like?
A: Google it.
Q8. Are there hybrid orbitals?
A: Yes! These are used in making bonds. You’d have seen them in chem bonding lessons.
Q9. How do you deduce electronic configuration from successive IE?
A: Please read my notes on Ionisation Energies Discussion
Q10. Why does repulsion for paired electrons matter less in s orbital than in p orbitals?
A: Please read my notes on Ionisation Energies Discussion
Q11. Can electron spins be changed?
A: Yes! This requires energy.
Q12. What is meant by orbitals become more diffuse?
A: The orbitals are larger and so the electron density is spread out and diffuse.
Q13. Are there remedial lessons for clueless pple?
A: we can work on that. But remedial lesson involve a lot of work on your part as well.
Q14. How is the colour change in chemical reactions brought about?
A: In a chemical reaction one compou8nd is transformed to another, and if they have different colours, there is a colour change! But why are compounds coloured? Very often its due to their electronic structure! Most of the time, electrons absorb light energy to transit from ground state to excited states. The energy and therefore colour of light absorbed depends on the particular electronic structure. Light that is not absorbed but reflected is what give the colours of compounds. Refer to qn12 on why beta carotene is orange.
Tuesday, March 07, 2006
Atomic structure Clarification Questions
A: Well keep in mind that the orbitals are simply areas where you have a 95% probability of finding the electron. The electron, being a very small particle, can actually behave like a wave (eg like light waves), which is one of the reasons why electron microscopes work (electrons instead of light is used!). Anyway, this is why we cannot pin the electron down to defined orbits, but rather in a space where the probability current is high. Basically to define the wave behaviour of an electron, the Schrodinger wave equation is used. Once you got that, you can use a probability flux equation (like a Gaussian curve) to describe the space its in.
This probability space derive from the equations defines the shape of the orbital itself.
The nasty bits of maths and physics can be found ->here <- For those that can’t stand the math and physics, just know the shapes of s p d orbitals k?
There is experimental evidence for the discovery of the orbitals. Its answered in Q13. But for all the bio enthusiasts, it is useful to know about the electron microscope. This is especially true if you are interested in viral work or work on cell organelles.
Q2. What new improvements can be made to the current model of the atom?
A: I dunno, but if I do, I probably can get a Nobel prize for that.
Q3. How can this knowledge be useful in our daily lives?
A:Well, it really depends on what your daily life is like. See, if you only like to watch tv and blog, you probably won’t use the knowledge. But know that this knowledge is what makes modern advances possible. For eg, medicine: without the electron microscope, it is impossible to gain our knowledge so far about viruses, and about cell organelles. You can see some interesting electron micrographs here.
So if you wanna be one of those people that improve our way of life and create new advances, you are going to need a good foundation in knowledge and knowledge skills. If you can’t even understand old discoveries, how are you going to make new ones?
Also, some very interesting properties of matter lie in seemingly trivial differences between s-orbitals and p-orbitals. For eg, Why is SiO2 a solid, with giant covalent structure, while CO2 a gas with simple molecular structure, when both are group 4 oxides? To answer this qn, you have to know how the orbitals are used in bonding which I will not go into right now. But if CO2 is a solid, there probably won’t be life on earth. Oh by the way, if the speed of light or mass of proton or electron is different, there probably won’t be a universe. Read Stephen Hawking’s “A Brief History of time” for some details. Nice book and not too much hardcore physics.
The understanding of the model of the atom paves the way for the understanding for much of the rest of chemistry. Such as structures and shapes of organic molecules, and the mechanism of how reactions work. For eg, organic molecules have certain structures which give them function and properties, but heir structure actually depends on how the atomic orbitals combine to form bonds. Another example: rearrangment reactions, a knowledge of atomic orbitals and molecular orbitals (formed from atomic orbitals) enables us to explain how and why rearrangement reactions take place. and rearrangement reactions are important in areas such as chemical synthesis (where we can make new chemical such as drugs) and also in biochemistry (many biosynthesis reactions involve them)
By the way, quite a bit of your A lvl stuff are century++ old discoveries.
Q4: Are there any other factors that influence attraction of electron to nucleus?
A: The main factors are always the i) nuclear charge, ii) shielding from inner electrons (depends on principle shell), followed by iii) penetration power of subshell iv) repulsion from electrons in the same shell/orbital)
Q5: Are there any flaws to the spdf atomic model?
A: You mean quantum mechanical model? Well not really, the QM model is most accurate and predicts and explains most observations we make, including bonding, emission and absorption spectra, electrical conductivity, chemical reactions, biochemical reactions including rearrangement. The only problem is that the description is rather complex. But then, nature is not always what it seems, and our little human brains find it challenging to understand nature.
Q6. I still do not understand the behaviour of 3d orbitals.
A; I do not understand your question. What behaviour?
Q7.How did democritus know for sure that matter is indestructible?
A: He didn’t! hah that is why the old theory of matter was incorrect! (as in atoms are not the smallest particles you can get. I guess he postulated this based on observations he could make at his time. With more advances mankind made, better theories were developed. So when you study, know that the method to develop knowledge is just as important.
Q8. What is the use of drawing orbitals?
A; So you can see what they look like (haha). Seriously, Q1-7 to get whats the point of knowing all this.
Q9. What is electron density?
A: Exactly what it says. Althought more accurately the statement should be electron probability density. It is the probability of finding an electron present in an area.
Q10. Which atom has the highes ionization energies?
A: Ne and F.
Q11. How do we know the electrons fill up different orbitals and shells?
A: 1. Schrodinger wave equation/( refer to Q1)
2. Ionisation energy experiments.
3. Atomic emission and absorption spectra.
Q12. Why do we need to know where to find an electron?
A: The idea was actually refine our idea structure of atoms. The old model of spherical orbits for example, just don’t cut it. For eg. The from the old model we cannot explain why molecules have certain shapes for example. And if molecules don’’t have certain shapes, then our universe would be messed up. Matter would look different. Biomelecules such as enzymes and DNA won’t be the same and won’t work.
Carrots won’t look orange!
I’m serious! Beta-carotene is orange, because it has lots of conjugated double bonds. Why does that make it orange? Because, atomic orbitals combine to form molecular orbitals. The conjugated pi molecular orbitals have an antibonding and bonding orbital that allows electrons to jump between them and absorb light energy in the process. The energy absorb happens to correspond to blue light energy, so only orange /red/yellow light is reflected.
Haha so to answer your question, why’d we want to know anything? So we can explain how everything works! So we can understand nature. And hopefully master over nature after that. See Q3.
Q13. How was the different energy levels found out?
A: Ionisation energy experiments were proof of different energy levels. Electrical discharge tubes were used to ionize gases. Modern methods use light energy(photons) to do the job more accurately. This method is called spectroscopy, and electrons from different orbitals interacted with photons differently. Using spectroscopy, emission and absorption spectra could be produced that allows us to deduce the existence of different orbitals.
Check out this paper on the determination of IE of CuO and FeO
Q14. Are the graphs for f orbitals the addition of s and d orbitals?
A: NO! Again pls refer to Q1
Q15: Applications of spin quantum no?
A: They are part of the quantum mechanical model of atoms.
Q16.: Is there anyway to calculate the exact location of an electron?
A: Not humanly possible at the moment. It appears nature doesn’t allow it.
Q17: Why do electron have different spins?
A: so that 2 electrons can occupy 1 orbital.
Q18: How fast do electrons orbit?
A: it depends, the large the orbital the faster the speed. For eg, f orbital electrons in gold atoms approach the speed of light. Incidentally, this actually explains why gold is such an inert metal.
Q19: Are there scientists who dispute this model? What are their arguments?
A: A good question! Unfortunately I don’t know the answer. First of all, very few mortals are brilliant enough to even completely understand quantum mechanics, much less dispute it. And because this model has worked so well, many critics have been effectively silenced… Any new model proposed would have to be just as rigorous and work even better
Q20: Why can’t there be standard rules with no exceptions?
A: Well, because this is nature. Remember science is man made, but it is a study of nature, which is not subject to our whims and wishes. Actually its because science is manmade that’s why there are so many rules, most of which are empirical and man made.
Just look at the octet rule debacle. Most of the time the many rules are due to Man’s incomplete, piecemeal understanding of nature. For eg.in the octet rule debacle, an understanding of energy and quantum mechanical model actually unifies things, such as why some elements can have more than an octet? Why does xenon form compounds even if if is noble gas?
Q21: why were the s p d f orbitals names as such?
A: You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the letter designations have nothing to do with the orbital shapes.
Spectroscopists associated transitions involving energy levels with different values with different groups of lines in the line spectra of the alkali metals. The line groups were called sharp, principal, diffuse, and fundamental. When the angular momentum quantum number was used to describe and explain these groups of lines, s became an abbreviation for = 0, p meant = 1, d meant = 2, and f meant = 3. For consistency, higher values of the angular momentum quantum numbers are designated alphabetically (g means = 4, h means = 5, and so on).
Q22: How were the different orbitals discovered?
A: Refer to Q13 and Q1
Q23: Why cant there be only s orbitals, why is there all the s p and d orbitals?
A: That’s nature. If things were so simple, the universe won’t have human beings. It will only have electrons and vacuum. Then we won’t be sitting around here to wonder about stuff.
Q24.”What is the effect on neutrons on deflection of nuclei in electric fields?
A: Neutrons add mass. So go figure.
Q25: How does IE increase across a period?
A: Across a period, generally 1. nuclear charge increase, 2. shielding effect stays constant, 3 therefore effective nuclear charge increases, 4 hence IE increases.
Q26: How can knowledge of atomic orbitals help me in making of new materials?
A: Nice question. Go read up on how atomic orbitals hybridize and combine to form molecular orbitals. Molecular orbitals are what makes bonds, and bonds are what make materials. You can start from here.
Q27. Does IE affect reactivity series?
A:Most definitely! For eg, potassium K is more reactive than sodium Na. Why? Because, K has a lower IE than Na and will lose its electron more readily. Later on, if you learn about f orbital effects, you will understand why gold is so inert.
Q28. Are you so free? What a long Q and A! A: Ugh, I’m too busy. But I take my work seriously, and so should you. I still manage to make time for family, friends, but I want to make sure my work is done well. Just don’t waste your time on unproductive things.
Sunday, March 05, 2006
you can always use a pair of tweezers
dismayed.
I've got a quite a few things to blog today. Like:
1) results of the Atomic structure quiz
2) shaving blades
3) table lamps
4) cab drivers.
I'm done marking the atomic structure quiz and analyzing results. They were very unimpressive. If you consider that the test is so easy, the results for some kids are awful, appalling and utterly disappointing. Please do not think this is some supra-ohmigosh-intelligent high level RJC test k. Its just a normal standard A lvl test. Sth like what I'd give in AC also. .. but its 1 am in the morn and I dun wanna go into the details. Oh but there are some students who did well too! And i'm going to post test statistics and a roll of honour on this blog soon.
moving on to shaving blades. Huh?? yep they R for shaving off the hair of those slackers that did so poorly in the quiz. eh hahaa ok as much as i wanna do that, I was kidding k. really kidding. some1 from moe may be reading this. I was KIDDING K! but maybe info can diffuse into those bald heads more efficiently? Its already hard enuff getting past those thick skulls, we really dun need the overgelled hair.
ok I'm KIDDING K!
rar thoughts of nice clean sharp shaving blades actually came floating around as I was rubbing my stubble. Can you imagine, i shave in the morning everyday, and by the afternoon, the shadow has grown out. by night its stubble. ugh what a waste of protein. i could really use all that protein somewhere else. but no, my face insists on growing hair! and I 've run out of shaving blades. the current one has gone blunt and i need to shave the next morning for work right?? so i turned round and ask my bro
me: hey lets go out for a drive, I need to buy some shaving blades and maybe get some sweets
bro: ehh.. you sure you can get your kind of blades? not all 7-11s have'em
me: i gas I'll have to try, its so irritating i run thru my blades so quickly
bro: well you can always use a pair of tweezers. just pull em all out and no shaving for weeks!
me:.....omg ugh... lemme use those tweezers on your
bros say the darndestdest things.
oh I think repeated use of shaving blades causes pimples. some1 should stardee that.
eh the table lamp and cab drivers are going to have to wait. nuff of blogging today.
IMPORTANT! read the following!!
Ok, we've been using, very loosely, penetration power and penetrating power. as in 3s orbital has more penetrating power vs 3p orbital.
I mean, if sth is highly penetrating, it'll achieve high penetration? non?
non. The more common usage is PENETRATING power.
So there be warned!!!!!~~~.. talk about penetrating, not penetration in the future.
Man, stuff on this blog is going really deep. and very hardcore.
Atomic structure strikes back aaaagain.
Ok i realised that the earlier post the link to the ionisation energy discussion couldnt work. Apparently I can't share out of my yahoo briefcase. So, i simply created another website to host the document. *tee hee*. This should work fine!
simply -->click here<--
:)
Saturday, March 04, 2006
look there! ------------------------------------------------------->
There's sth wrong with this blog.... what? the tag board... its all the waaaaaay at the bottom!
bleh I have no idea how to get it back up (i mean the tag board)
frustrated....
about that plus the non stop chinese techno music my bro is currently blasting from his hi fi.
Suddenly I feel like increasing the levels of entropy in my immediate neighbourhood.
><
whats entropy? go google it can?
The atomic structure strikes back
I've refined, compiled my set of notes on ionisation energy discussions and converted it to flash! Now if any of you out there can clue me in on how to upload files to share with people on the net and to link from here to that file easily, please let me know!
For now, as a temporary measure, I'm going to rely on yahoo briefcase.
Here's the link!
btw its a flash file, so you'd need to have macromedia flash plugin installed..
--> Click here <--
Have fun!
Thursday, March 02, 2006
Fallacy of the abused Octet
Say for example,

Now lets consider another situation
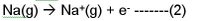
With the octet stability fallacy, most people will actually consider Na+ to be more stable than Na, or think that Na+ is easily and spontaneously formed.
But we know that for reaction 2 , ionization energy is required. So Na+(g) is higher in energy and is unstable. In fact it doesn’t want to be formed and we need to inject energy. So what of the stable octet of Na+ then?
Let us relook at reaction (1) This reaction is extremely exothermic and spontaneous. Why? Because the NaCl formed has very strong ionic bonds, and its formation releases energy. So its not Na+ that’s stable, but NaCl that’s stable.
Diagrammatically the energy changes of reaction (1)can be represented like:
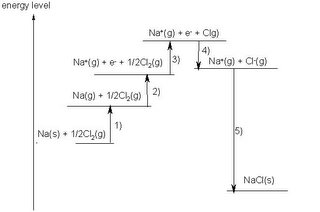

In other words, the driving force of the reaction is NOT the formation of the stable octet. It is the release of energy that drives this reaction (and all other reactions). There is no such thing as the desire to achieve a "stable octet" being the driving force of electron sharing or transfer.
Hence, the use of disruption of octet to justify large IE (eg of Ne)
or the formation of full octet to justify small IE (eg of Na) is incorrect.
Ne has a very large 1st IE compared to Na since its electron is taken from the 2nd shell which expereinces much less shielding, compared to Na's 3rd shell electron. The same is true for Na+ (it has no more 3rd shell electrons, only 2nd shell electrons left)
So the octet rule should be framed as tool to predict no of electrons that are transferred or shared.. that’s all it is.
So why does the octet rule work? What makes it predict rather accurately the number of electrons transferred? Investigating our NaCl example, we can question, why only Na+ is formed? Why not make Na2+?
The answer is :
- Successive IE increases, and
- removal of inner quantum shell electrons is very difficult
-since they experience very much less shielding.
-As a result Na loses its valence electron easily (small 1st IE) but the
-2nd inner shell electron is not easily removed (very large 2nd IE)
-So the formation of NaCl takes place, but not the formation of NaCl2. (refer to diagram above, making Na2+ will require so much energy that NaCl2 will be higher in energy and unstable)
The octet rule exists due to energetics! Therefore the octet rule is an empirical observation cannot be applied to explain ionization energy. It is in fact the other way around.
The regions round echo the sound
What an emotional ride. I felt such joy for some students, and also empathised with the tears of others.. very often I was lost for words.
First off I am mostly contented and happy and relieved. My classes all scored above average against others of their own subject combinations, both in terms of %As and mean subject grade.
most of the students that I have seen potential in them have managed to get their As and Bs. and some who were getting Fs actually passed nicely with a C. For them I thank God. ACJC also did very well this year, more 3 and 4,5,6,7,8 As, and 2 fellas with 9 distinctions (one of them the student council president, no less). Truely, the best is yet to be.
But there were one or two complacent individuals who refused throughout the two years to be consistent, to put in effort, to even attend classes, despite persuasion, encouragement, counselling, threats from me, and other tutors. They did badly. How do we react? Only that its a pity that their potential was wasted, and will continue to be so until they realise that potential, or natural talent is akin to a rough uncut diamond. Without the hardwork that goes into shaping the diamond, without the creative energies injected by a craftman, that diamond will never shine to the world. Hopefully then they will learn to put in that hardwork, discipline and perseverance, and work with their future mentors to unlock their potential.
All in all, I am glad I had a firm hand on the students that I had the privilege (or pain) to work with. While sometimes I had to be so strict, take unpopular action and risk being misunderstood, I think this day is a day when what matters become so clear. The students have grown stronger, more mature. Their mental capacity has expanded They've seen their hardwork pay off, and learned the value of discipline and perseverance. Their great potential is reflected on their results, and they are thankful for it. To be firm, to demand the highest standards from my students, and to have faith that they have potential, this is the best way to love them.
wow, I've rambled for so long! i'm so brain dead from all the excitement, all that catching up with old friends and ex students... so dazed now... some marking not done yet.. I'd better go!
Coming soon, the fallacy of the abused octet, and the atomic structure strikes back.
RJC class of 2005 also did very well; clinching the highest number of 4A's in 25 years.
I'm so... preoccupied now.... with paranoid thoughts of what I would write here 2 years from now, when the current batch of J1s in RJ gets their turn.