Say for example,

Now lets consider another situation
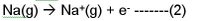
With the octet stability fallacy, most people will actually consider Na+ to be more stable than Na, or think that Na+ is easily and spontaneously formed.
But we know that for reaction 2 , ionization energy is required. So Na+(g) is higher in energy and is unstable. In fact it doesn’t want to be formed and we need to inject energy. So what of the stable octet of Na+ then?
Let us relook at reaction (1) This reaction is extremely exothermic and spontaneous. Why? Because the NaCl formed has very strong ionic bonds, and its formation releases energy. So its not Na+ that’s stable, but NaCl that’s stable.
Diagrammatically the energy changes of reaction (1)can be represented like:
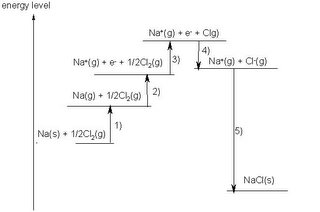

In other words, the driving force of the reaction is NOT the formation of the stable octet. It is the release of energy that drives this reaction (and all other reactions). There is no such thing as the desire to achieve a "stable octet" being the driving force of electron sharing or transfer.
Hence, the use of disruption of octet to justify large IE (eg of Ne)
or the formation of full octet to justify small IE (eg of Na) is incorrect.
Ne has a very large 1st IE compared to Na since its electron is taken from the 2nd shell which expereinces much less shielding, compared to Na's 3rd shell electron. The same is true for Na+ (it has no more 3rd shell electrons, only 2nd shell electrons left)
So the octet rule should be framed as tool to predict no of electrons that are transferred or shared.. that’s all it is.
So why does the octet rule work? What makes it predict rather accurately the number of electrons transferred? Investigating our NaCl example, we can question, why only Na+ is formed? Why not make Na2+?
The answer is :
- Successive IE increases, and
- removal of inner quantum shell electrons is very difficult
-since they experience very much less shielding.
-As a result Na loses its valence electron easily (small 1st IE) but the
-2nd inner shell electron is not easily removed (very large 2nd IE)
-So the formation of NaCl takes place, but not the formation of NaCl2. (refer to diagram above, making Na2+ will require so much energy that NaCl2 will be higher in energy and unstable)
The octet rule exists due to energetics! Therefore the octet rule is an empirical observation cannot be applied to explain ionization energy. It is in fact the other way around.
1 comment:
Thank you for clarifying this concept that you introduced to us in class. This entry really crystallises what you've told us the other day. Your layout is also very neat and nice! (Hope you actually read this comment)
Post a Comment