A: Well keep in mind that the orbitals are simply areas where you have a 95% probability of finding the electron. The electron, being a very small particle, can actually behave like a wave (eg like light waves), which is one of the reasons why electron microscopes work (electrons instead of light is used!). Anyway, this is why we cannot pin the electron down to defined orbits, but rather in a space where the probability current is high. Basically to define the wave behaviour of an electron, the Schrodinger wave equation is used. Once you got that, you can use a probability flux equation (like a Gaussian curve) to describe the space its in.
This probability space derive from the equations defines the shape of the orbital itself.
The nasty bits of maths and physics can be found ->here <- For those that can’t stand the math and physics, just know the shapes of s p d orbitals k?
There is experimental evidence for the discovery of the orbitals. Its answered in Q13. But for all the bio enthusiasts, it is useful to know about the electron microscope. This is especially true if you are interested in viral work or work on cell organelles.
Q2. What new improvements can be made to the current model of the atom?
A: I dunno, but if I do, I probably can get a Nobel prize for that.
Q3. How can this knowledge be useful in our daily lives?
A:Well, it really depends on what your daily life is like. See, if you only like to watch tv and blog, you probably won’t use the knowledge. But know that this knowledge is what makes modern advances possible. For eg, medicine: without the electron microscope, it is impossible to gain our knowledge so far about viruses, and about cell organelles. You can see some interesting electron micrographs here.
So if you wanna be one of those people that improve our way of life and create new advances, you are going to need a good foundation in knowledge and knowledge skills. If you can’t even understand old discoveries, how are you going to make new ones?
Also, some very interesting properties of matter lie in seemingly trivial differences between s-orbitals and p-orbitals. For eg, Why is SiO2 a solid, with giant covalent structure, while CO2 a gas with simple molecular structure, when both are group 4 oxides? To answer this qn, you have to know how the orbitals are used in bonding which I will not go into right now. But if CO2 is a solid, there probably won’t be life on earth. Oh by the way, if the speed of light or mass of proton or electron is different, there probably won’t be a universe. Read Stephen Hawking’s “A Brief History of time” for some details. Nice book and not too much hardcore physics.
The understanding of the model of the atom paves the way for the understanding for much of the rest of chemistry. Such as structures and shapes of organic molecules, and the mechanism of how reactions work. For eg, organic molecules have certain structures which give them function and properties, but heir structure actually depends on how the atomic orbitals combine to form bonds. Another example: rearrangment reactions, a knowledge of atomic orbitals and molecular orbitals (formed from atomic orbitals) enables us to explain how and why rearrangement reactions take place. and rearrangement reactions are important in areas such as chemical synthesis (where we can make new chemical such as drugs) and also in biochemistry (many biosynthesis reactions involve them)
By the way, quite a bit of your A lvl stuff are century++ old discoveries.
Q4: Are there any other factors that influence attraction of electron to nucleus?
A: The main factors are always the i) nuclear charge, ii) shielding from inner electrons (depends on principle shell), followed by iii) penetration power of subshell iv) repulsion from electrons in the same shell/orbital)
Q5: Are there any flaws to the spdf atomic model?
A: You mean quantum mechanical model? Well not really, the QM model is most accurate and predicts and explains most observations we make, including bonding, emission and absorption spectra, electrical conductivity, chemical reactions, biochemical reactions including rearrangement. The only problem is that the description is rather complex. But then, nature is not always what it seems, and our little human brains find it challenging to understand nature.
Q6. I still do not understand the behaviour of 3d orbitals.
A; I do not understand your question. What behaviour?
Q7.How did democritus know for sure that matter is indestructible?
A: He didn’t! hah that is why the old theory of matter was incorrect! (as in atoms are not the smallest particles you can get. I guess he postulated this based on observations he could make at his time. With more advances mankind made, better theories were developed. So when you study, know that the method to develop knowledge is just as important.
Q8. What is the use of drawing orbitals?
A; So you can see what they look like (haha). Seriously, Q1-7 to get whats the point of knowing all this.
Q9. What is electron density?
A: Exactly what it says. Althought more accurately the statement should be electron probability density. It is the probability of finding an electron present in an area.
Q10. Which atom has the highes ionization energies?
A: Ne and F.
Q11. How do we know the electrons fill up different orbitals and shells?
A: 1. Schrodinger wave equation/( refer to Q1)
2. Ionisation energy experiments.
3. Atomic emission and absorption spectra.
Q12. Why do we need to know where to find an electron?
A: The idea was actually refine our idea structure of atoms. The old model of spherical orbits for example, just don’t cut it. For eg. The from the old model we cannot explain why molecules have certain shapes for example. And if molecules don’’t have certain shapes, then our universe would be messed up. Matter would look different. Biomelecules such as enzymes and DNA won’t be the same and won’t work.
Carrots won’t look orange!
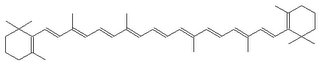
I’m serious! Beta-carotene is orange, because it has lots of conjugated double bonds. Why does that make it orange? Because, atomic orbitals combine to form molecular orbitals. The conjugated pi molecular orbitals have an antibonding and bonding orbital that allows electrons to jump between them and absorb light energy in the process. The energy absorb happens to correspond to blue light energy, so only orange /red/yellow light is reflected.
Haha so to answer your question, why’d we want to know anything? So we can explain how everything works! So we can understand nature. And hopefully master over nature after that. See Q3.
Q13. How was the different energy levels found out?
A: Ionisation energy experiments were proof of different energy levels. Electrical discharge tubes were used to ionize gases. Modern methods use light energy(photons) to do the job more accurately. This method is called spectroscopy, and electrons from different orbitals interacted with photons differently. Using spectroscopy, emission and absorption spectra could be produced that allows us to deduce the existence of different orbitals.
Check out this paper on the determination of IE of CuO and FeO
Q14. Are the graphs for f orbitals the addition of s and d orbitals?
A: NO! Again pls refer to Q1
Q15: Applications of spin quantum no?
A: They are part of the quantum mechanical model of atoms.
Q16.: Is there anyway to calculate the exact location of an electron?
A: Not humanly possible at the moment. It appears nature doesn’t allow it.
Q17: Why do electron have different spins?
A: so that 2 electrons can occupy 1 orbital.
Q18: How fast do electrons orbit?
A: it depends, the large the orbital the faster the speed. For eg, f orbital electrons in gold atoms approach the speed of light. Incidentally, this actually explains why gold is such an inert metal.
Q19: Are there scientists who dispute this model? What are their arguments?
A: A good question! Unfortunately I don’t know the answer. First of all, very few mortals are brilliant enough to even completely understand quantum mechanics, much less dispute it. And because this model has worked so well, many critics have been effectively silenced… Any new model proposed would have to be just as rigorous and work even better
Q20: Why can’t there be standard rules with no exceptions?
A: Well, because this is nature. Remember science is man made, but it is a study of nature, which is not subject to our whims and wishes. Actually its because science is manmade that’s why there are so many rules, most of which are empirical and man made.
Just look at the octet rule debacle. Most of the time the many rules are due to Man’s incomplete, piecemeal understanding of nature. For eg.in the octet rule debacle, an understanding of energy and quantum mechanical model actually unifies things, such as why some elements can have more than an octet? Why does xenon form compounds even if if is noble gas?
Q21: why were the s p d f orbitals names as such?
A: You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the letter designations have nothing to do with the orbital shapes.
Spectroscopists associated transitions involving energy levels with different values with different groups of lines in the line spectra of the alkali metals. The line groups were called sharp, principal, diffuse, and fundamental. When the angular momentum quantum number was used to describe and explain these groups of lines, s became an abbreviation for = 0, p meant = 1, d meant = 2, and f meant = 3. For consistency, higher values of the angular momentum quantum numbers are designated alphabetically (g means = 4, h means = 5, and so on).
Q22: How were the different orbitals discovered?
A: Refer to Q13 and Q1
Q23: Why cant there be only s orbitals, why is there all the s p and d orbitals?
A: That’s nature. If things were so simple, the universe won’t have human beings. It will only have electrons and vacuum. Then we won’t be sitting around here to wonder about stuff.
Q24.”What is the effect on neutrons on deflection of nuclei in electric fields?
A: Neutrons add mass. So go figure.
Q25: How does IE increase across a period?
A: Across a period, generally 1. nuclear charge increase, 2. shielding effect stays constant, 3 therefore effective nuclear charge increases, 4 hence IE increases.
Q26: How can knowledge of atomic orbitals help me in making of new materials?
A: Nice question. Go read up on how atomic orbitals hybridize and combine to form molecular orbitals. Molecular orbitals are what makes bonds, and bonds are what make materials. You can start from here.
Q27. Does IE affect reactivity series?
A:Most definitely! For eg, potassium K is more reactive than sodium Na. Why? Because, K has a lower IE than Na and will lose its electron more readily. Later on, if you learn about f orbital effects, you will understand why gold is so inert.
Q28. Are you so free? What a long Q and A! A: Ugh, I’m too busy. But I take my work seriously, and so should you. I still manage to make time for family, friends, but I want to make sure my work is done well. Just don’t waste your time on unproductive things.
No comments:
Post a Comment